3D cell culture scaffolds designed as hard polymer fibre constructs:
Such design provides a favourable environment for cell attachment and proliferation. The round fibre and pore morphology follows a random structure of natural extracellular matrix (as compared to straight/aligned fibre and filament structures). Large pores allow for an efficient cell distribution throughout the entire scaffold height.
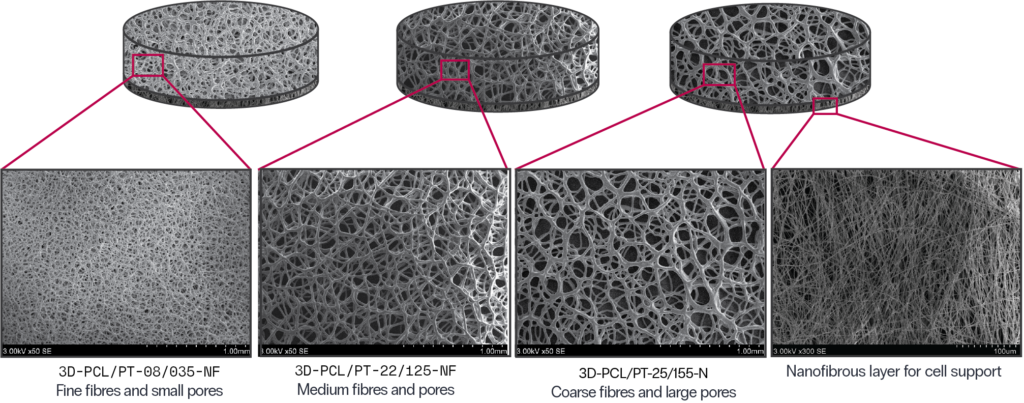
Specifications:
Construction: 3D, 2-layer (nanofibre bottom)
Basis polymer: PCL, plasma-treated
SKU: 3D-PCL/PT-08/035-NF
Fibre diameter (mean) 8 μm
Pore diameter (mean) 35 μm
SKU: 3D-PCL/PT-22/125-NF
Fibre diameter (mean) 22 μm
Pore diameter (mean) 125 μm
SKU: 3D-PCL/PT-25/155-NF
Fibre diameter (mean) 25 μm
Pore diameter (mean) 155 μm
Features
• Tunable morphology
• Cut-to-size
• Easy imaging
• UV-sterilized and ready to use
• Compatible with most current 2D assays
Applications
• Drug screening
• Environmental cytotoxicity
• Tissue engineering
• Organ-on-chip models
Modifications
• Wetting properties
• Cold plasma surface activation
• Attachment of functional molecules (growth factors)
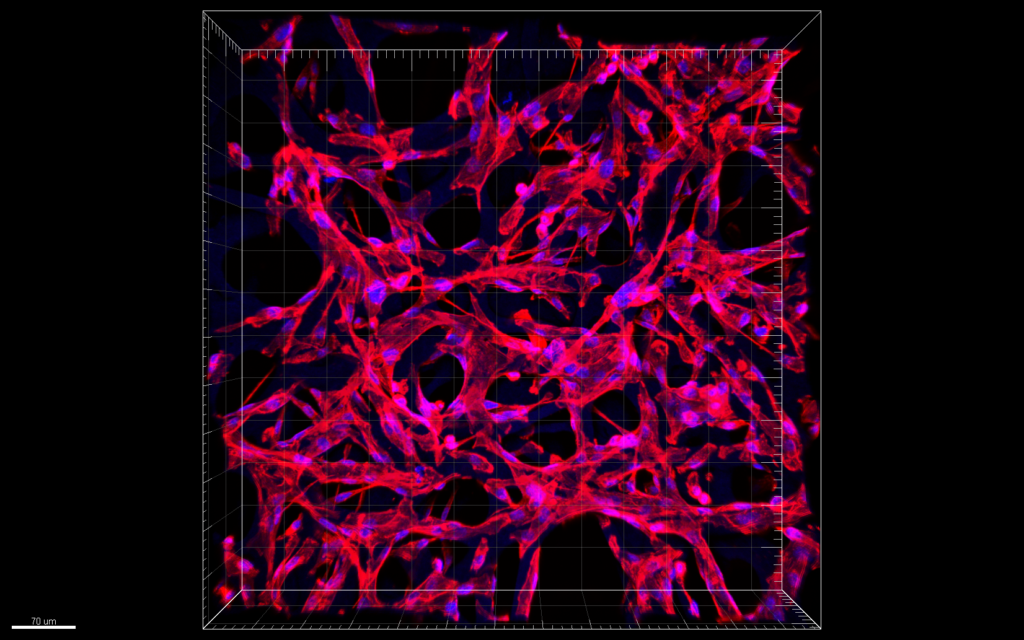
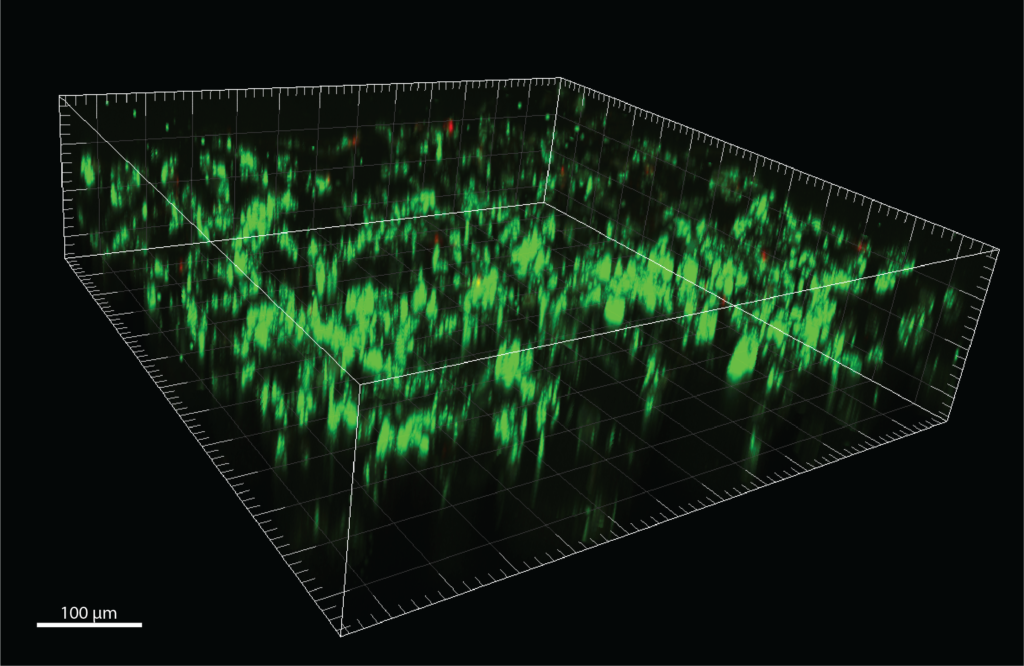
Confocal microscopy images of MDA-MB-231 cells in scaffold
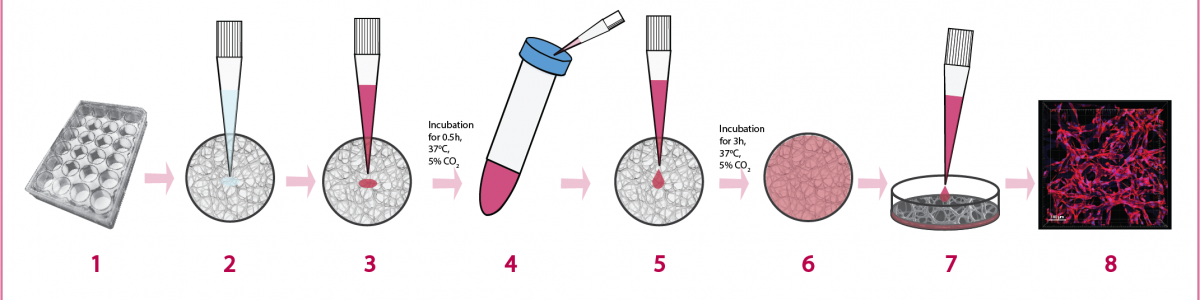
Common protocols for 2D cell cultures can also be adapted when working with 3D cell cultures.
Scaffolds are compatible with both assays.
Recent application: https://doi.org/10.1016/j.bej.2022.108531
Ciuzas D., Krugly, E., Petrikaite, V. Fibrous 3D printed poly(ɛ)caprolactone tissue engineering scaffold for in vitro cell models. Biochemical Engineering Journal, Volume 185, July 2022, 108531
